P(v) intermediate-mediated E1cB elimination for the synthesis of glycals†
Abstract
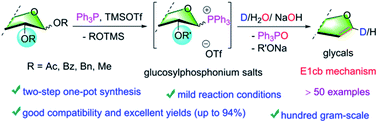
Glycals are highly versatile and useful building blocks in the chemistry of carbohydrate and natural products. However, the practical synthesis of glycals remains a long-standing and mostly unsolved problem in synthetic chemistry. Herein, we present an unprecedented approach to make a variety of glycals using phosphonium hydrolysis-induced, P(V) intermediate-mediated E1cB elimination. The method provides a highly efficient, practical and scalable strategy for the synthesis of glycals with good generality and excellent yields. Furthermore, the strategy was successfully applied to late-stage modification of complex drug-like molecules. Additionally, the corresponding 1-deuterium-glycals were produced easily by simple tBuONa/D2O-hydrolysis–elimination. Mechanistic investigations indicated that the oxaphosphorane intermediate-mediated E1cB mechanism is responsible for the elimination reaction.


 Please wait while we load your content...
Please wait while we load your content...