Design and synthesis of stable four-coordinated benzotriazole-borane with tunable fluorescence emission†
Abstract
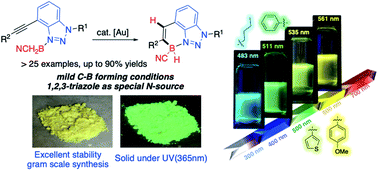
A new class of stable four-coordinated benzotriazole-borane compounds was developed via gold-catalyzed alkyne hydroboration. The application of polymeric (BH2CN)n reagent gave the formation of cyano-amine-boranes (CAB) complexes with less basic N-heterocyclic amines and anilines. Various new CABs were investigated in catalytic hydroboration to synthesize N–B cycles. The 1,2,3-benzotriazoles were identified as the only feasible N-source, giving the four coordinated borane N–B cycles (BTAB) in excellent yields (up to 90%) with good functional group tolerability. This new class of polycyclic N–B compounds showed excellent stability toward acid, base, high temperature, and photo-irradiation. The facile synthesis, excellent stability, strong and tunable fluorescence emission make BTAB interesting new fluorescent probes for future chemical and biological applications.


 Please wait while we load your content...
Please wait while we load your content...