Directing group assisted rhodium catalyzed meta-C–H alkynylation of arenes†
Abstract
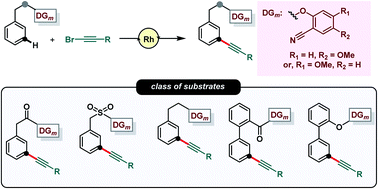
Site-selective C–H alkynylation of arenes to produce aryl alkynes is a highly desirable transformation due to the prevalence of aryl alkynes in various natural products, drug molecules and in materials. To ensure site-selective C–H functionalization, directing group (DG) assisted C–H activation has been evolved as a useful synthetic tool. In contrast to DG-assisted ortho-C–H activation, distal meta-C–H activation is highly challenging and has attracted significant attention in recent years. However, developments are majorly focused on Pd-based catalytic systems. In order to diversify the scope of distal meta-C–H functionalization, herein we disclosed the first Rh(I) catalyzed meta-C–H alkynylation protocol through the inverse Sonogashira coupling reaction. The protocol is compatible with various substrate classes which include phenylacetic acids, hydrocinnamic acids, 2-phenyl benzoic acids, 2-phenyl phenols, benzyl sulfonates and ether-based scaffolds. The post-synthetic modification of meta-alkynylated arenes is also demonstrated through DG-removal as well as functional group interconversion.


 Please wait while we load your content...
Please wait while we load your content...