Copper-catalyzed radical cascade reaction of simple cyclobutanes: synthesis of highly functionalized cyclobutene derivatives†
Abstract
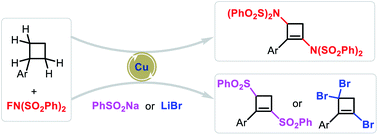
Cyclobutenes as versatile and highly valuable synthons have been widely applied in synthesis. Although various methods for their synthesis have been well established, new strategies for the construction of the cyclobutene skeleton from simple substrates are still highly desirable. Starting from simple cyclobutanes, the construction of the cyclobutene skeleton especially introducing multiple functional groups simultaneously had never been achieved. Here, we developed a novel radical cascade strategy for the synthesis of highly functionalized cyclobutenes directly from cyclobutanes involving rare cleavage of four or five C–H bonds and formation of two C–N/C–S or three C–Br bonds. With copper as catalyst and N-fluorobenzenesulfonimide (NFSI) as oxidant, a wide range of diaminated, disulfonylated and tribrominated cyclobutene derivatives were efficiently synthesized.


 Please wait while we load your content...
Please wait while we load your content...