A (μ-hydrido)diborane(4) anion and its coordination chemistry with coinage metals†
Abstract
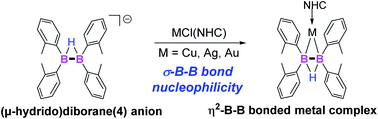
A tetra(o-tolyl) (μ-hydrido)diborane(4) anion 1, an analogue of [B2H5]− species, was facilely prepared through the reaction of tetra(o-tolyl)diborane(4) with sodium hydride. Unlike common sp2–sp3 diborane species, 1 exhibited a σ-B–B bond nucleophilicity towards NHC-coordinated transition-metal (Cu, Ag, and Au) halides, resulting in the formation of η2-B–B bonded complexes 2 as confirmed by single-crystal X-ray analyses. Compared with 1, the structural data of 2 imply significant elongations of B–B bonds, following the order Au > Cu > Ag. DFT studies show that the diboron ligand interacts with the coinage metal through a three-center-two-electron B–M–B bonding mode. The fact that the B–B bond of the gold complex is much prolonged than the related Cu and Ag compounds might be ascribed to the superior electrophilicity of the gold atom.


 Please wait while we load your content...
Please wait while we load your content...