Direct visible-light-induced synthesis of P-stereogenic phosphine oxides under air conditions†
Abstract
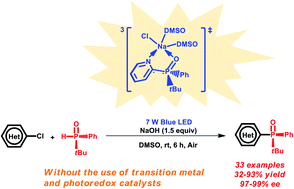
Over the past two decades, visible-light-induced transformations have been regarded as being among the most environmentally benign and powerful strategies for constructing complex molecules and diverse synthetic building blocks in organic synthesis. However, the development of efficient photochemical processes for assembling enantiomerically pure molecules remains a significant challenge. Herein, we describe a simple and efficient visible-light-induced C–P bond forming reaction for the synthesis of P-chiral heteroaryl phosphine oxides in moderate to high yields with excellent ee values (97–99% ee). Even in the absence of transition metal or photoredox catalysts, a variety of P-chiral heteroaryl phosphine oxides, including chiral diphosphine oxide 41, have been directly obtained under air conditions. Density functional theory (DFT) calculations have shown that the reaction involves intersystem crossing and single electron transfer to give a diradical intermediate under visible light irradiation.
- This article is part of the themed collection: In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...