Unraveling the reactivity of a cationic iminoborane: avenues to unusual boron cations†
Abstract
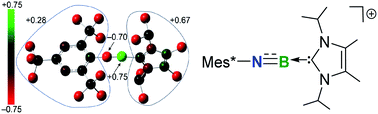
A cationic terminal iminoborane [Mes*N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B ← IPr2Me2][AlBr4] (3+[AlBr4]−) (Mes* = 2,4,6-tri-tert-butylphenyl and IPr2Me2 = 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene) has been synthesized and characterized. The employment of an aryl group and N-heterocyclic carbene (NHC) ligand enables 3+[AlBr4]− to exhibit both B-centered Lewis acidity and BN multiple bond reactivities, thus allowing for the construction of tri-coordinate boron cations 5+–12+. More importantly, initial reactions involving coordination, addition, and [2 + 3] cycloadditions have been observed for the cationic iminoborane, demonstrating the potential to build numerous organoboron species via several synthetic routes.
B ← IPr2Me2][AlBr4] (3+[AlBr4]−) (Mes* = 2,4,6-tri-tert-butylphenyl and IPr2Me2 = 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene) has been synthesized and characterized. The employment of an aryl group and N-heterocyclic carbene (NHC) ligand enables 3+[AlBr4]− to exhibit both B-centered Lewis acidity and BN multiple bond reactivities, thus allowing for the construction of tri-coordinate boron cations 5+–12+. More importantly, initial reactions involving coordination, addition, and [2 + 3] cycloadditions have been observed for the cationic iminoborane, demonstrating the potential to build numerous organoboron species via several synthetic routes.


 Please wait while we load your content...
Please wait while we load your content...