Selective electrochemical capture and release of uranyl from aqueous alkali, lanthanide, and actinide mixtures using redox-switchable carboranes†
Abstract
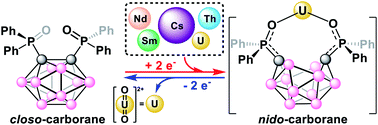
We report the selective electrochemical biphasic capture of the uranyl cation (UO22+) from mixed-metal alkali (Cs+), lanthanide (Nd3+, Sm3+), and actinide (Th4+, UO22+) aqueous solutions to an organic, 1,2-dichloroethane (DCE), phase using the ortho-substituted nido-carborane anion, [1,2-(Ph2PO)2-1,2-C2B10H10]2− (POCb2−). The reduced POCb2− is generated by electrochemical reduction of the closo-carborane, POCb, prior to mixing with the aqueous mixed-metal solution. Subsequent UO22+ release from the captured product, [UO2(POCb)2]2−, was performed by galvanostatic bulk electrolysis of the DCE phase and back-extraction of UO22+ to a fresh aqueous phase. The selective capture and release of UO22+ was confirmed by combined ICP-OES and NMR spectral analyses of the aqueous and organic phases, respectively, against the newly synthesized nido-carborane complexes, [[CoCp*2][Cs(POCb)]]2, [CoCp*2]3[Nd(POCb)3], [CoCp*2]3[Sm(POCb)3], and [CoCp*2]2[Th(POCb)3].


 Please wait while we load your content...
Please wait while we load your content...