Diverse saturated heterocycles from a hydroacylation/conjugate addition cascade†
Abstract
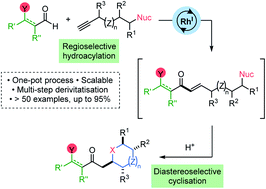
Rhodium-catalyzed hydroacylation using alkynes substituted with pendant nucleophiles, delivers linear α,β-unsaturated enone intermediates with excellent regioselectivity. These adducts are used to construct a broad range of diversely substituted, saturated O-, N- and S-heterocycles in a one-pot process. Judicious choice of cyclisation conditions enabled isolation of O-heterocycles with high levels of diastereoselectivity. A variety of derivatisation reactions are also performed, generating functionalised hydroacylation products. This sequence serves as a general approach for the synthesis of fully saturated heterocycles.


 Please wait while we load your content...
Please wait while we load your content...