Fully fused boron-doped polycyclic aromatic hydrocarbons: their synthesis, structure–property relationships, and self-assembly behavior in aqueous media†
Abstract
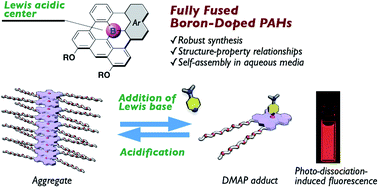
Planarized triarylboranes are attracting increasing attention not only as models of boron-doped graphenes, but also as promising materials for organic optoelectronics. In particular, polycyclic aromatic hydrocarbon (PAH) skeletons with embedded boron atom(s) in the inner positions are of importance in light of their high chemical stability and π-stacking ability derived from their planar geometries. Herein, we disclose a robust synthesis of such fully fused boron-doped PAHs and their self-assembly behavior in aqueous media to explore their potential utility in biological applications. The synthesis using in situ-generated planar diarylboranes as a key precursor afforded a series of fully fused boron-doped PAHs, even including an amphiphilic derivative with hydrophilic side chains. These compounds exhibited red emission in solution, and slight structural modification resulted in increased fluorescence brightness. While these compounds showed relatively low Lewis acidity compared to their partially ring-fused counterparts, their Lewis acidities were slightly increased in polar solvents compared to those in nonpolar solvents. In addition, their B–N Lewis acid–base adducts, even those with a strong, charge-neutral Lewis base such as N,N-dimethylaminopyridine (DMAP), exhibited photo-dissociation behavior in the excited state. The amphiphilic derivative showed significant spectral changes with increased water content in DMSO/H2O mixed media and formed sheet-like aggregates. The disassembly and assembly processes of the aggregates were externally controlled by the addition of DMAP and an acid, accompanied by a change in the fluorescence intensity.
- This article is part of the themed collection: Most popular 2022 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...