Enantioselective one-carbon expansion of aromatic rings by simultaneous formation and chromoselective irradiation of a transient coloured enolate†
Abstract
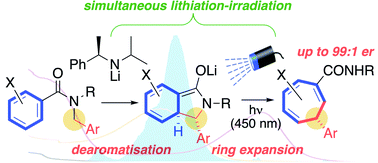
Enantioenriched seven-membered carbocycles are motifs in many molecules of structural and biological interest. We report a simple, practical, transition metal-free and mechanistically unusual method for the enantioselective synthesis of substituted cycloheptatrienes. By forming a coloured enolate with an appropriate absorption band and selectively irradiating in situ, we to initiate a tandem, asymmetric anionic and photochemical ring expansion of readily accessible N-benzylbenzamides. The cascade of reactions leading to the products entails enantioselective benzylic deprotonation with a chiral lithium amide, dearomatizing cyclization of the resulting configurationally defined organolithium to give an extended amide enolate, and photochemically induced formal [1,7]-sigmatropic rearrangement and 6π-electrocyclic ring-opening – the latter all evidently being stereospecific – to deliver enantioenriched cycloheptatrienes with embedded benzylic stereocentres.


 Please wait while we load your content...
Please wait while we load your content...