Metal-free, visible-light induced enantioselective three-component dicarbofunctionalization and oxytrifluoromethylation of enamines via chiral phosphoric acid catalysis†
Abstract
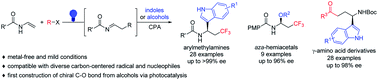
Using diverse carbon-centered radical precursors and electron-rich (hetero)aromatics and alcohols as nucleophiles, a visible-light driven chiral phosphoric acid (CPA) catalyzed asymmetric intermolecular, three-component radical-initiated dicarbofunctionalization and oxytrifluoromethylation of enamines was developed, which provides a straightforward access to chiral arylmethylamines, aza-hemiacetals and γ-amino acid derivatives with excellent enantioselectivity. As far as we know, this is the first example of constructing a chiral C–O bond using simple alcohols via visible-light photocatalysis. Chiral phosphoric acid played multiple roles in the reaction, including controlling the reaction stereoselectivity and promoting the generation of radical intermediates by activating Togni's reagent. Mechanistic studies also suggested the importance of the N–H bond of the enamine and indole for the reactions.


 Please wait while we load your content...
Please wait while we load your content...