Hypervalent iodine-mediated β-difluoroalkylboron synthesis via an unusual 1,2-hydrogen shift enabled by boron substitution†
Abstract
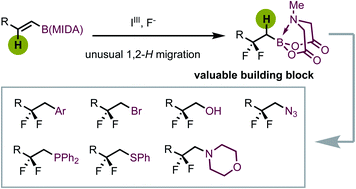
β-Difluoroalkylborons, featuring functionally important CF2 moiety and synthetically valuable boron group, have great synthetic potential while remaining synthetically challenging. Herein we report a hypervalent iodine-mediated oxidative gem-difluorination strategy to realize the construction of gem-difluorinated alkylborons via an unusual 1,2-hydrogen migration event, in which the (N-methyliminodiacetyl) boronate (BMIDA) motif is responsible for the high regio- and chemoselectivity. The protocol provides facile access to a broad range of β-difluoroalkylborons under rather mild conditions. The value of these products was demonstrated by further transformations of the boryl group into other valuable functional groups, providing a wide range of difluorine-containing molecules.


 Please wait while we load your content...
Please wait while we load your content...