Atropoenantioselective palladaelectro-catalyzed anilide C–H olefinations viable with natural sunlight as sustainable power source†
Abstract
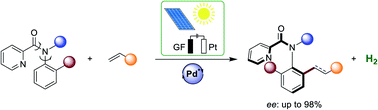
Enantioselective electrocatalyzed transformations represent a major challenge. We herein achieved atropoenantioselective pallada-electrocatalyzed C–H olefinations and C–H allylations with high efficacy and enantioselectivity under exceedingly mild reaction conditions. With (S)-5-oxoproline as the chiral ligand, activated and non-activated olefins were suitable substrates for the electro-C–H activations. Dual catalysis was devised in terms of electro-C–H olefination, along with catalytic hydrogenation. Challenging enantiomerically-enriched chiral anilide scaffolds were thereby obtained with high levels of enantio-control in the absence of toxic and cost-intensive silver salts. The resource-economy of the transformation was even improved by directly employing renewable solar energy.


 Please wait while we load your content...
Please wait while we load your content...