Solvent directed chemically divergent synthesis of β-lactams and α-amino acid derivatives with chiral isothiourea†
Abstract
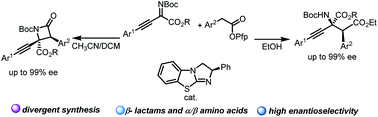
A protocol for the chemically divergent synthesis of β-lactams and α-amino acid derivatives with isothiourea (ITU) catalysis by switching solvents was developed. The stereospecific Mannich reaction occurring between imine and C(1)-ammonium enolate generated zwitterionic intermediates, which underwent intramolecular lactamization and afforded β-lactam derivatives when DCM and CH3CN were used as solvents. However, when EtOH was used as the solvent, the intermediates underwent an intermolecular esterification reaction, and α-amino acid derivatives were produced. Detailed mechanistic experiments were conducted to prove that these two kinds of products came from the same intermediates. Furthermore, chemically diversified transformations of β-lactam and α-amino acid derivatives were achieved.


 Please wait while we load your content...
Please wait while we load your content...