OvoAMtht from Methyloversatilis thermotolerans ovothiol biosynthesis is a bifunction enzyme: thiol oxygenase and sulfoxide synthase activities†
Abstract
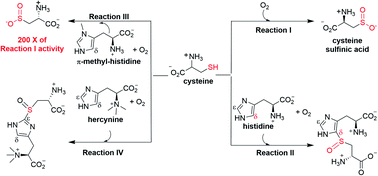
Mononuclear non-heme iron enzymes are a large class of enzymes catalyzing a wide-range of reactions. In this work, we report that a non-heme iron enzyme in Methyloversatilis thermotolerans, OvoAMtht, has two different activities, as a thiol oxygenase and a sulfoxide synthase. When cysteine is presented as the only substrate, OvoAMtht is a thiol oxygenase. In the presence of both histidine and cysteine as substrates, OvoAMtht catalyzes the oxidative coupling between histidine and cysteine (a sulfoxide synthase). Additionally, we demonstrate that both substrates and the active site iron's secondary coordination shell residues exert exquisite control over the dual activities of OvoAMtht (sulfoxide synthase vs. thiol oxygenase activities). OvoAMtht is an excellent system for future detailed mechanistic investigation on how metal ligands and secondary coordination shell residues fine-tune the iron-center electronic properties to achieve different reactivities.


 Please wait while we load your content...
Please wait while we load your content...