Rapid and column-free syntheses of acyl fluorides and peptides using ex situ generated thionyl fluoride†
Abstract
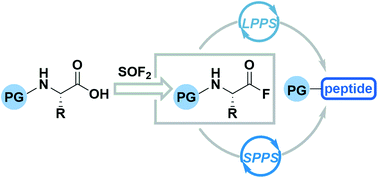
Thionyl fluoride (SOF2) was first isolated in 1896, but there have been less than 10 subsequent reports of its use as a reagent for organic synthesis. This is partly due to a lack of facile, lab-scale methods for its generation. Herein we report a novel protocol for the ex situ generation of SOF2 and subsequent demonstration of its ability to access both aliphatic and aromatic acyl fluorides in 55–98% isolated yields under mild conditions and short reaction times. We further demonstrate its aptitude in amino acid couplings, with a one-pot, column-free strategy that affords the corresponding dipeptides in 65–97% isolated yields with minimal to no epimerization. The broad scope allows for a wide range of protecting groups and both natural and unnatural amino acids. Finally, we demonstrated that this new method can be used in sequential liquid phase peptide synthesis (LPPS) to afford tri-, tetra-, penta-, and decapeptides in 14–88% yields without the need for column chromatography. We also demonstrated that this new method is amenable to solid phase peptide synthesis (SPPS), affording di- and pentapeptides in 80–98% yields.


 Please wait while we load your content...
Please wait while we load your content...