Organocatalytic enantioselective SN1-type dehydrative nucleophilic substitution: access to bis(indolyl)methanes bearing quaternary carbon stereocenters†
Abstract
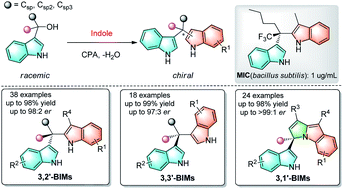
A highly general and straightforward approach to access chiral bis(indolyl)methanes (BIMs) bearing quaternary stereocenters has been realized via enantioconvergent dehydrative nucleophilic substitution. A broad range of 3,3′-, 3,2′- and 3,1′-BIMs were obtained under mild conditions with excellent efficiency and enantioselectivity (80 examples, up to 98% yield and >99 : 1 er). By utilizing racemic 3-indolyl tertiary alcohols as precursors of alkyl electrophiles and indoles as C–H nucleophiles, this organocatalytic strategy avoids pre-activation of substrates and produces water as the only by-product. Mechanistic studies suggest a formal SN1-type pathway enabled by chiral phosphoric acid catalysis. The practicability of the obtained enantioenriched BIMs was further demonstrated by versatile transformation and high antimicrobial activities (3al, MIC: 1 μg mL−1).


 Please wait while we load your content...
Please wait while we load your content...