Non-metal boron atoms on a CuB12 monolayer as efficient catalytic sites for urea production†
Abstract
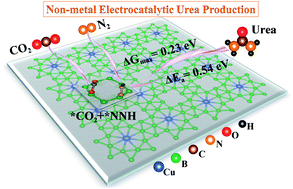
An electrocatalytic C–N coupling reaction to convert CO2 and N2 into urea under mild conditions has been proposed to be a promising alternative experimentally, but the development of highly stable, low-cost and high-performance non-metal catalytic sites remains rare and challenging. Herein, a global-minimum CuB12 monolayer with superior stability has been identified based on first-principles computations, and the most significant finding is that the CuB12 monolayer possesses the best catalytic activity among the reported urea catalysts thermodynamically and kinetically. All possible reaction pathways to form urea (NH2CONH2) starting from the CO2 molecule and N2 molecule, including the CO2 pathway, OCOH pathway, CO pathway, NCON pathway and mixed pathway, as well as the kinetic energy barriers of six possible C–N coupling reactions are systematically investigated. Non-metal B atoms at the midpoint of the edges of the squares act as excellent catalytic sites with a limiting potential of urea production of 0.23 V through the CO2 pathway and OCOH pathway and the lowest kinetic energy barrier of C–N bond formation (0.54 eV) through the reaction *CO + *NHNH → *NHCONH. Therefore, this study not only identifies the first non-metal B catalytic sites for urea formation, but also perfects the reaction mechanism to convert CO2 and N2 into urea, which could provide great guiding significance to explore other high-performance urea catalysts.


 Please wait while we load your content...
Please wait while we load your content...