EDC-promoted one-step synthesis of teriflunomide at the industrial scale†
Abstract
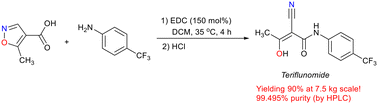
Promoted by EDC, the reaction of 5-methylisoxazole-4-carboxylic acid with 4-(trifluoromethyl)aniline could produce the medicine teriflunomide in over 90% yield. In comparison with known methods, this novel production process was reduced to only one step of reaction, free of a chlorinating agent. The reaction could be scaled up to produce 7.5 kg of teriflunomide (yielding 90%, with 99.495% purity), providing a concise and practical method for the synthesis of this well-known medicine. The unique chemical structure of EDC containing an amine moiety endows the molecule with unique properties for promoting the reaction.


 Please wait while we load your content...
Please wait while we load your content...