Catalyst and reactor design considerations for selective production of acids by oxidative cleavage of alkenes and unsaturated fatty acids with H2O2†
Abstract
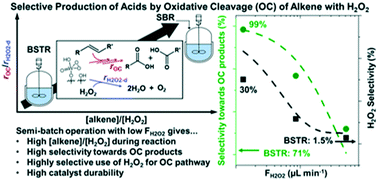
Oxidative cleavage of alkenes and unsaturated fatty acids with hydrogen peroxide gives an efficient and sustainable process to obtain mono- and di-acids for polymers and lubricants with fewer safety risks and less environmental impact than processes that utilize ozone or other inorganic oxidizers (e.g., permanganate, dichromate, etc.). Guided by insight into the mechanisms for competing reaction pathways (i.e., epoxidation of alkene on W–(η2-O2) complexes vs. H2O2 decomposition) and the apparent kinetics derived from kinetic experiments, here, we postulate that W-based heterogeneous catalysts can provide high performance and stable operations at low H2O2 concentrations. Semi-batch reactors with continuous introduction of H2O2 solutions offer the means to maintain low H2O2 concentrations while providing sufficient quantities of H2O2 to satisfy the reaction stoichiometry. We derived simple kinetic model equations for the epoxidation, ring-opening, oxidative cleavage, and oxidation steps and fit theses equations to batch experimental data to obtain kinetic parameters. This kinetic model describes the concentration profiles of reactant, oxidant, and products well as shown by agreement with experimental data. Further predictions of the optimal H2O2 feed rate for semi-batch operation utilized by the proposed rate expressions and the reactor design equations suggest that low H2O2 feed rate increases selectivity towards oxidative cleavage products and selective use of H2O2 for oxidative cleavage pathway. Comparisons of oxidative cleavage of 4-octene in batch and semi-batch reactors show that semibatch reactors with optimized molar feed rates of H2O2 increased oxidative cleavage product selectivities (76% to 99%; with an increase in butyric acid selectivity from 1% to 55%) and H2O2 selectivity (3% to 30%). In addition, semibatch reaction conditions used avoid H2O2-mediated dissolution of W-atoms from the catalyst. Analysis of these findings suggest that solid oxide catalysts will be effective for continuous oxidative cleavage reactions if deployed within fixed-bed reactors that allow for distributed introduction of reactants and therefore low in situ concentrations of H2O2.


 Please wait while we load your content...
Please wait while we load your content...