Flow synthesis of an α-amino boronic ester as a key precursor of bortezomib drug†
Abstract
The flow synthesis of the optically active α-amino boronate precursor of the bortezomib drug is described, including a key diastereoselective Matteson rearrangement. This reaction sequence provides an efficient, prompt and valuable strategy for the preparation of bortezomib (<30 min total residence time over 3 steps for the flow synthesis of the protected L-boroleucine).
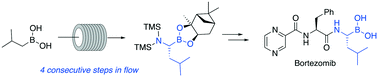


 Please wait while we load your content...
Please wait while we load your content...