Plasma nitrogen fixation in the presence of a liquid interface: role of OH radicals†
Abstract
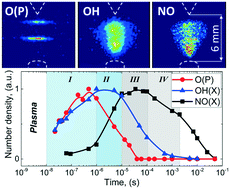
Physical backgrounds of a nitrogen fixation process in a nanosecond pulsed discharge in the presence of a plasma/liquid interface are reported. The role of OH radicals and O atoms in NO formation is experimentally revealed for the plasma operating in a single pulse mode and high-frequency burst. The kinetic curves demonstrate NO radical formation up to 10's of μs that well agrees with the observed OH radical behavior. The measurements in the single mode illustrate the sequence of the gas-phase chemistry that can be simplified as follows: electrons → N2(electronically excited) → O(D) → OH → NO. The increase of the operating frequency up to 100 kHz does not affect the pathway of NO formation in high electrical field plasma (such as dielectric barrier discharges or ns-pulsed plasma studied here). Independently of the frequency, the NO generation takes place through the extended Zeldovich mechanism dominantly driven by OH radicals because of the presence of the plasma/liquid interface whereas the O atoms' contribution in direct NO formation is low.


 Please wait while we load your content...
Please wait while we load your content...