Sulphur poisoning, water vapour and nitrogen dilution effects on copper-based catalyst dynamics, stability and deactivation during CO2 reduction reactions to methanol†
Abstract
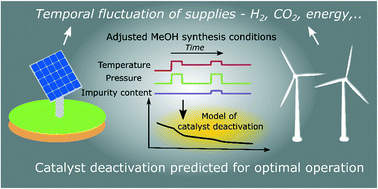
To reduce CO2 emissions, a flexible process operation for chemical methanol synthesis may be required as the supply of renewable energy-based feedstocks fluctuates. Analysis for the determination of the changing conditions of the long-term activity of catalysts is therefore important for efficient industrial production. A commercial Cu/ZnO/Al2O3 catalyst and five other materials (CuBaTiOX, CuCaTiOX, CuCeAlOX, CuSrAlOX and CuSrTiOX), prepared using solution combustion methods, were tested at two different pressures (p), followed by ageing at high temperature (T) jumps to show the effects of sensitivity. Surfaces were characterized by N2 physisorption, scanning electron microscopy, coupled with energy dispersive spectroscopy (SEM-EDS), and crystallographic X-ray diffraction (XRD) with additional structural Rietveld refinement. Stability reduction mechanisms were assessed, a model was developed with the applied relative partial p of reaction product species as input, and, for CuCeAlOX, it was demonstrated that the kinetics of deactivation is related to a unified H2O gage p distribution, while excluding the correlations of other four prevalent gases (hydrogen, carbon dioxide, carbon monoxide and methanol). An activity decrease can be predicted. Interestingly, synthesized SrCO3-containing mixtures exhibited a lesser loss of initial methanol synthesis activity at 50 bar than at 20 bar during time-on-stream increased T application. In addition, the activity relationship of the catalysts with N2 and H2S poisoning was described. A linear performance differentiation as a function of the amount of H2S impurity was observed, presented and mechanistically modelled. Carbon capture and utilisation technologies, power-to-liquid and e-fuels, will often require (realistic) non-steady state dynamics, which we herein simulate catalytically.
- This article is part of the themed collection: ISCRE26 & APCRE9: Celebrating Chemical Reaction Engineering


 Please wait while we load your content...
Please wait while we load your content...