Green and sustainable synthesis of poly(δ-valerolactone) with a TBD catalyzed ring-opening polymerization reaction†
Abstract
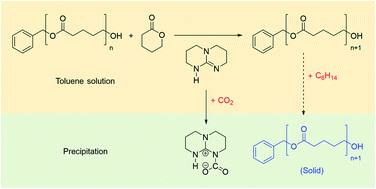
Ring-opening polymerization (ROP) of lactones catalyzed by 1,5,7-triazabicyclo[4,4,0]decane-5-ene (TBD) is a highly efficient method for synthesizing aliphatic polyester materials. In order to create a green and sustainable synthetic route for poly(δ-valerolactone) with a narrow molecular weight distribution, we propose CO2 as the terminator instead of conventional organic acids, which are difficult to remove from the production solution. A flow ROP reactor for δ-valerolactone is employed to enhance the mass transfer of CO2 and implement the ROP reaction continuously. In addition to the quenching method, n-hexane is proposed as the antisolvent of the production solution to precipitate poly(δ-valerolactone) from toluene instead of normally used methanol, which works as an initiator of ROP that influences the molecular weight of polyesters as an impurity of the reaction solution. Owing to the strong hydrophobicity and volatility of n-hexane, the anhydrous toluene and residual δ-valerolactone have strong potential to be recycled via distillation separation.


 Please wait while we load your content...
Please wait while we load your content...