Kinetic study of o-nitrotoluene nitration in a homogeneously continuous microflow†
Abstract
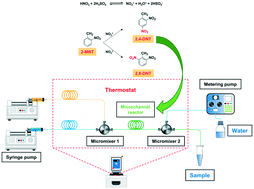
o-Nitrotoluene nitration can produce 2,4-dinitrotoluene and 2,6-dinitrotoluene simultaneously, which is also the common side-reaction of toluene mononitration. Kinetic study is helpful to deepen the understanding of nitration and control the reaction better. A homogeneously continuous microflow system was constructed to determine the kinetic parameters of 2-MNT nitration. A complete kinetic model was established and all kinetic parameters were obtained. In general, the observed reaction rate will increase with increasing H2SO4 mass fraction. However, when the H2SO4 mass fraction increases to a certain value, the observed reaction rate decreases. This is the first time to report the maximum observed reaction rate constant corresponding to the H2SO4 mass fraction at different temperatures in this work. Compared to previous reports on this reaction, the system provides more accurate kinetic parameters and can be developed into a reliable platform for the kinetic study of similar reactions.


 Please wait while we load your content...
Please wait while we load your content...