Extraction of alumina from high-alumina fly ash by ammonium sulfate: roasting kinetics and mechanism
Abstract
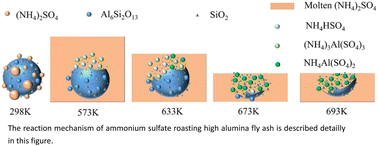
The recycling of aluminum is commonly an important step to achieve the high value-added utilization of fly ash, which is a kind of solid waste generated from coal-fired power plants. In this study, high-alumina fly ash was efficiently activated by ammonium sulfate method and the alumina was efficiently extracted. The effects of roasting temperature, roasting time, and ammonium sulfate/high-alumina fly ash mass ratio on the leaching rate of alumina were fully analyzed, and the roasting kinetics and reaction mechanism in the roasting process were discussed. The experimental results showed that the leaching rate of alumina in the roasted material achieved 93.57% with the roasting temperature of 673 K, the roasting time of 60 min, and the mass ratio of ammonium sulfate to high-alumina fly ash of 6 : 1. The roasting kinetics showed that the reaction between high-alumina fly ash and ammonium sulfate was controlled by internal diffusion, the apparent activation energy was 37.40 kJ mol−1, which accorded with the reaction kinetic equation 1 − 2x/3 − (1 − x)2/3 = 2.9546 exp[−37 400/(RT)]t. The reaction mechanism showed that the aluminum and structural damage mullite in the high-alumina fly ash reacted with molten ammonium sulfate to form (NH4)3Al(SO4)3 and NH4Al(SO4)2. Finally, (NH4)3Al(SO4)3 was transformed into NH4Al(SO4)2 with the increase of temperature.


 Please wait while we load your content...
Please wait while we load your content...