Cis–trans isomerization of dimethyl 2,3-dibromofumarate
Abstract
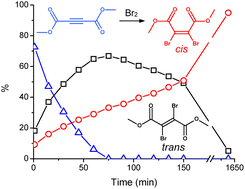
The isomerization of dimethyl 2,3-dibromofumarate in chloroform solutions was investigated by the combination of nuclear magnetic resonance (NMR) and density functional theory (DFT) calculations. The bromination of dimethyl acetylenedicarboxylate leading to dimethyl 2,3-dibromofumarate produces the trans isomer initially, which however converts into the more stable cis isomer. The conversion from trans to cis is spontaneous and greatly accelerated by light.


 Please wait while we load your content...
Please wait while we load your content...