Organocatalytic diastereo- and enantioselective conjugate addition of pyrazol-3-ones to 3-trifluoroethylidene oxindoles with a newly developed squaramide catalyst†
Abstract
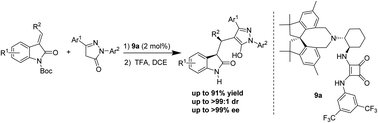
An efficient organocatalytic conjugated addition reaction of pyrazol-3-ones with 3-trifluoroethylidene oxindoles has been developed for the synthesis of enantioenriched triflouromethylated indolin-2-ones bearing adjacent tertiary chiral centers in good yields and good to excellent diastereo- and enantioselectivities. The use of a newly developed chiral spirobiindane-derived squaramide catalyst is essential in achieving high diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...