A novel procedure for the synthesis of borylated quinolines and its application in the development of potential boron-based homeodomain interacting protein kinase 2 (HIPK2) inhibitors†
Abstract
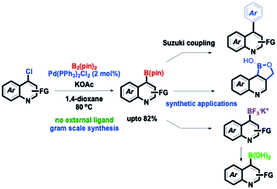
Herein, we demonstrate a Pd catalyzed C-4 borylation of structurally complex chloroquinolines with bis(pinacolato)diboron under relatively simple and efficient conditions. Moreover, the borylated quinolines were converted into oxaborole, trifluoroborate salt and boronic acid and also rendered in the Suzuki reaction successfully. The method was also applied for the synthesis of potential boron-based homeodomain interacting protein kinase 2 (HIPK2) inhibitors. The strategy opens up new avenues for the functionalization of quinolines as potential probes and pharmacological agents for future biomedical research.


 Please wait while we load your content...
Please wait while we load your content...