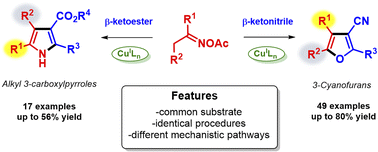
Divergent copper-catalyzed syntheses of 3-carboxylpyrroles and 3-cyanofurans from O-acetyl oximes and β-ketoesters/nitriles†
Abstract
The reaction between O-acetyl oximes and β-ketoesters/nitriles catalyzed by copper generated polysubstituted pyrroles and furans, respectively, under redox–neutral reaction conditions. Using this protocol, pyrroles or furans could be obtained simply by choosing an appropriate active methylene compound. Although both transformations occur essentially under the same reaction conditions, control experiments indicated that they follow different mechanistic pathways.


 Please wait while we load your content...
Please wait while we load your content...