Enhancing mechanism of arsenic(iii) adsorption by MnO2-loaded calcined MgFe layered double hydroxide†
Abstract
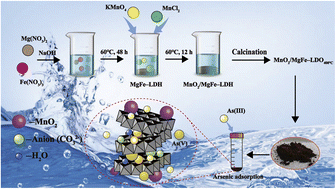
The use of MnO2/MgFe-layered double hydroxide (MnO2/MgFe-LDH) and MnO2/MgFe-layered double oxide (MnO2/MgFe-LDO400 °C) for arsenic immobilization from the aqueous medium is the subject of this research. Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy were used to characterise MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C. Based on our developed method, MnO2 was spread on the clay composites' surfaces in the form of a chemical bond. The clay composite exhibited a good adsorption effect on arsenic. The experimental findings fit the pseudo-second-order model well, indicating that the chemisorption mechanism played a significant role in the adsorption process. Furthermore, the Freundlich model suited the adsorption isotherm data of all adsorbents well. The recycling experiment showed that MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C exhibited good stability and reusability. In summary, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C are promising for developing processes for efficient control of the pollutant arsenic.


 Please wait while we load your content...
Please wait while we load your content...