Synthesis and evolution of physicochemical properties of new pharmaceutically active ionic liquids – tetracainium salicylate and tetracainium ibuprofenate†
Abstract
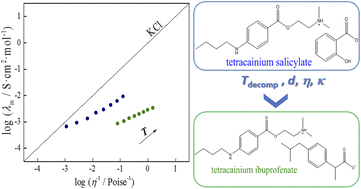
Tetracainium salicylate and tetracainium ibuprofenate were synthesized as active pharmaceutical ingredient ionic liquids (API-ILs). These ILs represent a combination of a drug for local anaesthesia (tetracaine) and nonsteroidal anti-inflammatory drugs (salicylic acid and ibuprofen). After IL synthesis, spectroscopic investigations were performed using infrared and nuclear magnetic resonance spectroscopy to confirm their structures. Differential scanning calorimetry and thermogravimetric analysis determined the obtained thermal behaviour of the ionic liquids. Experimental density, viscosity, and electrical conductivity measurements were performed in a wide temperature range to understand the interactions occurring in the obtained pharmaceutically active ionic liquids. All experimental values were well-fitted by the empirical equations. According to the theoretical calculations, weaker interactions of tetracaine with ibuprofenate than with salicylate are found, ascribed to the decreasing molecular symmetry, weakened hydrogen bonding, and increasing steric hindrance of ibuprofenate's alkyl chain.


 Please wait while we load your content...
Please wait while we load your content...