Study of the β-oxygen effect in the Barton–McCombie reaction for the total synthesis of (4R,5R)-4-hydroxy-γ-decalactone (Japanese orange fly lactone): a carbohydrate based approach†
Abstract
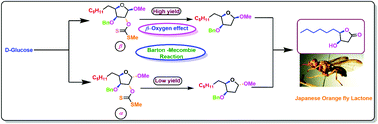
Efficient and facile synthesis of Japanese orange fly lactone (1) was achieved from a commercially available D-glucose by investigating the Barton–McCombie reaction with furanose anomeric isomers (12α, β) with an overall yield of 12.6%. During the course of this synthesis, the β-oxygen effect was discovered in the deoxygenation step at the C-3 position using the Barton–McCombie reaction, where the substrate allows the effect to operate in one of the isomers but not in the other. Under the same reaction conditions, xanthate derived from the β-furanose isomer affords a high yield of deoxygenated product, whereas the α-isomer produces a very low yield. The key transformations used were Wittig olefination, TEMPO mediated oxidation, and Barton–McCombie deoxygenation, resulting in a concise total synthesis of Japanese orange fly lactone (1). Our success will allow for further biological studies of this natural product, as well as opportunities for developing new potentially promising pheromones.


 Please wait while we load your content...
Please wait while we load your content...