Roles of cysteine in the structure and metabolic function of Mycobacterium tuberculosis CYP142A1†
Abstract
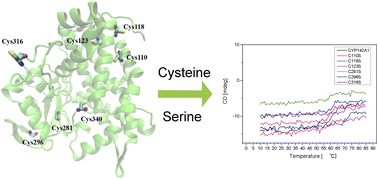
CYP142A1 is a cytochrome P450 (CYP) enzyme expressed in Mycobacterium tuberculosis (Mtb), which supports the growth of Mtb H37Rv relying on cholesterol, in the absence of CYP125A1. Since cysteine residues usually play a fundamental role in maintaining the structure and function of CYP enzymes, in this study, we aimed to determine the potential biochemical functions of six cysteine residues except for the heme-binding cysteine in the amino acid sequence of recombinant Mtb CYP142A1 by replacing each one using site-directed mutagenesis. Recombinant CYP142A1 mutants were heterologously expressed, purified, and analyzed using ESI-MS, far-UV CD spectroscopy, UV-vis spectrophotometric titration, and metabolic function assays. Substitution of the cysteine residues caused various effects on the structure and function of CYP142A1. Separate substitution of the six cysteine residues resulted in numerous changes in the secondary structure, expression level, substrate-binding ability, inhibitor-binding ability, thermal stability and oxidation efficiency of the enzyme. These results contribute to our understanding of the biochemical roles of cysteine residues in the structure and function of Mtb CYP enzymes, especially their effects on the structure and function of CYP142A1.


 Please wait while we load your content...
Please wait while we load your content...