Cationic palladium(ii)-catalyzed synthesis of substituted pyridines from α,β-unsaturated oxime ethers†
Abstract
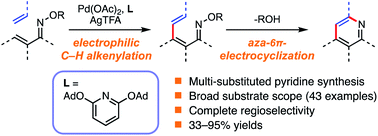
An efficient method for the synthesis of multi-substituted pyridines from β-aryl-substituted α,β-unsaturated oxime ethers and alkenes via Pd-catalyzed C–H activation has been developed. The method, using Pd(OAc)2 and a sterically hindered pyridine ligand, provides access to various multi-substituted pyridines with complete regioselectivity. Mechanistic studies suggest that the pyridine products are formed by Pd-catalyzed electrophilic C–H alkenylation of α,β-unsaturated oxime followed by aza-6π-electrocyclization. The utility of this method is showcased by the synthesis of 4-aryl-substituted pyridine derivatives, which are difficult to synthesize efficiently using previously reported Rh-catalyzed strategies with alkenes.


 Please wait while we load your content...
Please wait while we load your content...