Intermolecular hydrogen bonds between catechin and theanine in tea: slow release of the antioxidant capacity by a synergetic effect†
Abstract
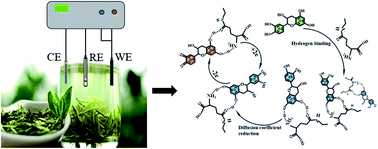
The health benefits of drinking tea stem from it being rich in polyphenols and other physiologically-active substances. Thus, exploring the synergistic effect between polyphenols and a variety of physiologically-active substances can contribute to our understanding of how tea benefits health. In this work, we have studied the interactions between catechin and theanine, exploring the synergetic antioxidant mechanism of the two molecules. Electrochemical characterization results showed that the oxidation peak current of catechin decreased gradually with the concentration of theanine, which is due to theanine spontaneously binding to catechin through intermolecular hydrogen bonds and forming molecular clusters via two hydrogen bonds. The binding constant is 4.75 at room temperature. The molecular clusters reduce the diffusion coefficient of catechin in solution, leading to the slow release of its antioxidant capacity (ability to effectively inhibit free radical oxidation reactions). Density functional theory calculations were also performed and verified the binding behavior. In identifying the synergistic effect between catechin and theanine on the antioxidant capacity of tea, this study adds to our understanding of the efficacy of tea polyphenols.


 Please wait while we load your content...
Please wait while we load your content...