Calculation of a temperature–volume phase diagram of water to inform the study of isochoric freezing down to cryogenic temperatures
Abstract
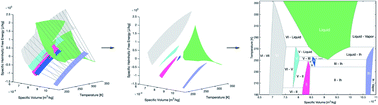
Phase diagrams are integral to the application and interpretation of materials thermodynamics, and none is more ubiquitous than the common temperature–pressure diagram of water and its many icy phases. Inspired by recent advances in isochoric thermodynamics, we here employ a simple convex hull approach to efficiently calculate an updated temperature–volume phase diagram for water and five of its solid polymorphs from existing Helmholtz free energy data. We proceed to highlight fundamental similarities between this T–V diagram and conventional binary temperature–concentration (T–x) diagrams, provide the volume coordinates of a variety of three-phase invariant reactions (e.g. “confined” or “volumetric” eutectics, peritectics, etc.) that occur amongst the phases of pure water under isochoric or confined conditions, and calculate the phase fraction evolution of ice Ih with temperature along multiple isochores of interest to experimental isochoric freezing. This work provides a requisite baseline upon which to extend the study of isochoric freezing to cryogenic temperatures, with potential applications in thermodynamic metrology, cryovolcanism, and cryopreservation.


 Please wait while we load your content...
Please wait while we load your content...