Structural elucidation of hexavalent Cr adsorbed on surfaces and bulks of Fe3O4 and α-FeOOH†
Abstract
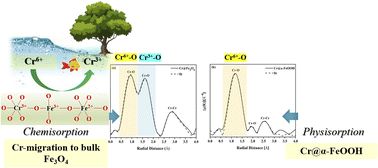
Magnetite (Fe3O4) and goethite (α-FeOOH) were synthesized via a hydrothermal approach and utilized as adsorbents for Cr6+ removal in an aqueous medium. The typical crystal structures of the synthesized Fe3O4 and α-FeOOH were confirmed by XRD and TEM. Fe3O4 in a spherical shape with a surface area of 32 m2 g−1 was established. While α-FeOOH had a rod-like form with a larger surface area of 84 m2 g−1. Cr6+ removal in an aqueous solution was studied in various conditions to evaluate thermodynamic and kinetic parameters. The adsorption isotherms on both adsorbents fit the Langmuir model indicating monolayer adsorption. Fe3O4 showed a better adsorption ability than α-FeOOH even though it had a lower surface area. XAS and XPS analysis strongly evidenced the production of stable Cr3+ species of Fe(1−x)CrxOOH and Fe(3−x)CrxO4 by Cr6+ reduction and migration processes into the bulk structure. Thus, the existence of stable Cr-species in Fe3O4 structure strongly affected Cr-adsorption ability rather than the surface area of the adsorbent. However, the precipitated Cr2O3 and HCrO4− molecules electrostatically adsorbed on the outer surface of α-FeOOH without bulk transformation. The presence of physisorbed FeO–HCrO4 species on α-FeOOH led to low reducibility and adsorption capability of Cr6+.


 Please wait while we load your content...
Please wait while we load your content...