Programing a cyanide-free transformation of aldehydes to nitriles and one-pot synthesis of amides through tandem chemo-enzymatic cascades†
Abstract
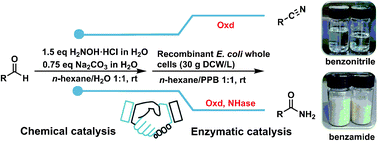
Nitriles are broadly applied to synthesize pharmaceuticals, agrochemicals, and materials because of their versatile transformation. Although various methods have been developed for introducing a nitrile group into organic molecules, most of them entail the use of highly toxic chemicals, transition metals, or harsh conditions. In this work, we reported a greener chemo-enzymatic cascade to synthesize alky and aryl nitriles from readily accessible aldehydes, that were further transformed into corresponding amides via an artificial enzyme cascade. A biphasic reaction system was designed to bridge chemical synthesis and enzymatic catalysis through simple phase separation. The biphasic system mainly perfectly avoided the inactivation of hydroxylamine on aldoxime dehydratase from Pseudomonas putida (OxdF1) and nitrile hydratase from Aurantimonas manganoxydans ATCC BAA-1229 (NHase1229). For the synthesis of various nitriles, moderate isolation yields of approximately 60% were obtained by the chemo-enzymatic cascade. Interestingly, two seemingly conflicting reactions of dehydration and hydration were sequentially proceeded to synthesize amides by the synergistic catalysis of OxdF1 and NHase1229 in E. coli cells. An isolation yield of approximately 62% was achieved for benzamide at the one-liter scale. In addition, the shuttle transport of substrates and products between two phases is convenient for the product separation and n-hexane recycling. Thus, the chemo-enzymatic cascade shows a potential application in the cyanide-free and large-scale synthesis of nitriles and amides.


 Please wait while we load your content...
Please wait while we load your content...