Nanomagnetic macrocyclic Schiff-base–Mn(ii) complex: an efficient heterogeneous catalyst for click approach synthesis of novel β-substitued-1,2,3-triazoles†
Abstract
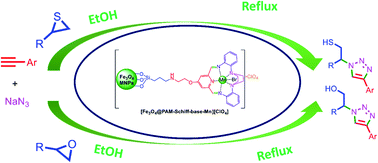
In the present work, a novel symmetrical 15-membered macrocyclic Schiff base complex of manganese was prepared using the reaction of the synthetic 2,6-diacetylpyridine functionalized Fe3O4 MNPs with 2,2-(piperazine-1,4-diyl)dianiline and Mn(II) bromide salt via a template approach. The resulting [Fe3O4@PAM–Schiff-base–Mn][ClO4] heterogenized complex was characterized using FT-IR, XRD, BET, TGA, EDX, Xray-mapping, SEM, TEM and VSM analysis. To demonstrate proof of concept, Huisgen 1,3-dipolar cycloaddition synthesis of 1,2,3-triazoles was selected to evaluate the activity and reusability of the catalyst. The ethanol as a green solvent proved to be an excellent reaction medium for this synthesis. Yields of up to 100% were obtained in some cases. Significantly, as demonstrated, [Fe3O4@PAM–Schiff-base–Mn][ClO4] catalyst was recycled for 8 cycles without losing catalytic activity under the optimized reaction conditions. The hot filtration and ICP-OES tests ratified that there was no leaching of metal during the catalytic reaction, indicating the heterogeneous manner of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...