Systematic study of physicochemical and electrochemical properties of carbon nanomaterials
Abstract
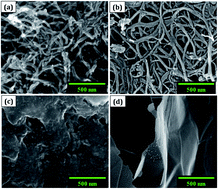
Carbon nanomaterials exhibit exceptional properties and broad horizon applications, where graphene is one of the most popular allotropes of this family due to its astounding performance in every stratum vis-à-vis other classical materials. The large surface area of 2630 m2 g−1, high electrical conductivity, and electron mobility of non-toxic graphene nanomaterials serve as the building blocks for supercapacitor studies. In this article, comparative studies are carried out between electrochemically exfoliated graphene sheets (GSs), solvothermally synthesized graphene quantum dots (GQDs) and acid refluxed carbon nanotubes (CNTs) as an energy storage electrode nanomaterial through cyclic voltammetry (CV). The electrochemical properties of the materials are well correlated with the physicochemical characteristics obtained from Raman, Fourier-transform infrared, and absorption spectroscopy. Thin GSs (0.8–1 nm) and small size (6–10 nm) GQDs fabricated by using laboratory-grade 99% purity graphite rods resulted in promising low-cost materials at mass scale as compared to conducting CNTs. The 0D graphene quantum dots proved to be an excellent energy electrode material in an alkaline electrolyte solution compared to other carbon nanomaterials. The distinct characteristic features of GQDs, like superior electrical properties, large surface area, and abundant active sites make them an ideal candidate for utilization in supercapacitors. The GQDs exhibited an enhanced specific capacitance of 113 F g−1 in 6 mol L−1 KOH through cyclic voltammetry.


 Please wait while we load your content...
Please wait while we load your content...