Structure–antitumor activity relationship of hybrid acetogenins focusing on connecting groups between heterocycles and the linker moiety†
Abstract
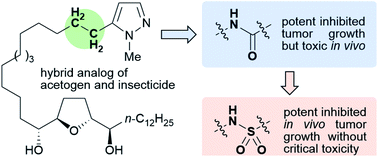
We studied hybrid molecules of annonaceous acetogenins and mitochondrial complex I-inhibiting insecticides to develop a novel anticancer agent. A structure–antitumor activity relationship study focusing on the connecting groups between the heterocycles and the linker moiety bearing the tetrahydrofuran moiety was conducted. Eleven hybrid acetogenins with 1-methylpyrazole instead of γ-lactone were synthesized and their growth inhibitory activities against 39 human cancer cell lines were evaluated. The nitrogen atom at the 2′-position of the linker moiety was essential for inhibiting cancer growth. The 1-methylpyrazole-5-sulfonamide analog showed potent growth inhibition of NCI-H23, a human lung cancer cell line, in a xenograft mouse assay without critical toxicity. Hence, the results of this study may pave the way for the development of novel anticancer agents, with both selective and broad anticancer activities.


 Please wait while we load your content...
Please wait while we load your content...