The NKT cell TCR repertoire can accommodate structural modifications to the lipid and orientation of the terminal carbohydrate of iGb3†
Abstract
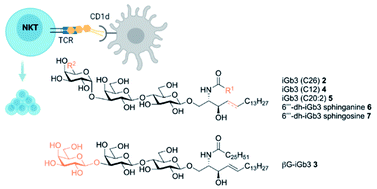
Isoglobotrihexosylceramide (iGb3) is a known NKT cell agonist, however the specific interactions required to trigger NKT cell TCR activation in response to this mammalian glycolipid are not fully understood. Here we report the synthesis of 1,3-β-Gal-LacCer (βG-iGb3) that displays a β-linked terminal sugar. βG-iGb3 activated NKT cells to a similar extent as iGb3 with a terminal α-linkage, indicating that the conformation of the terminal sugar residue of iGb3 is not essential to facilitate NKT cell TCR recognition. In addition, the immunological activity of four recently described iGb3 analogues with modifications to their terminal sugar or lipid backbone were also investigated. These iGb3 analogues all induced NKT cell proliferation, with IL-13 the predominate cytokine detected. This highlights the ability of the NKT cell TCR to accommodate variations in iGb3-based glycolipids and suggests that undiscovered NKT cell ligands may exist within the lacto-series of mammalian glycosphingolipids.


 Please wait while we load your content...
Please wait while we load your content...