Identification of a novel ene reductase from Pichia angusta with potential application in (R)-levodione production†
Abstract
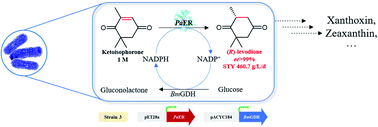
Asymmetric reduction of electronically activated alkenes by ene reductases (ERs) is an attractive approach for the production of enantiopure chiral products. Herein, a novel FMN-binding ene reductase (PaER) from Pichia angusta was heterologously expressed in Escherichia coli BL21(DE3), and the recombinant enzyme was characterized for its biocatalytic properties. PaER displayed optimal activity at 40 °C and pH 7.5, respectively. The purified enzyme was quite stable below 30 °C over a broad pH range of 5.0–10.0. PaER was identified to have a good ability to reduce the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of various α,β-unsaturated compounds in the presence of NADPH. In addition, PaER exhibited a high reduction rate (kcat = 3.57 s−1) and an excellent stereoselectivity (>99%) for ketoisophorone. Engineered E. coli cells harboring PaER and glucose dehydrogenase (for cofactor regeneration) were employed as biocatalysts for the asymmetric reduction of ketoisophorone. As a result, up to 1000 mM ketoisophorone was completely and enantioselectively converted to (R)-levodione with a >99% ee value in a space–time yield of 460.7 g L−1 d−1. This study provides a great potential biocatalyst for practical synthesis of (R)-levodione.
C bond of various α,β-unsaturated compounds in the presence of NADPH. In addition, PaER exhibited a high reduction rate (kcat = 3.57 s−1) and an excellent stereoselectivity (>99%) for ketoisophorone. Engineered E. coli cells harboring PaER and glucose dehydrogenase (for cofactor regeneration) were employed as biocatalysts for the asymmetric reduction of ketoisophorone. As a result, up to 1000 mM ketoisophorone was completely and enantioselectively converted to (R)-levodione with a >99% ee value in a space–time yield of 460.7 g L−1 d−1. This study provides a great potential biocatalyst for practical synthesis of (R)-levodione.


 Please wait while we load your content...
Please wait while we load your content...