Rapid detection of donor-dependent photocatalytic hydrogen evolution by NMR spectroscopy†
Abstract
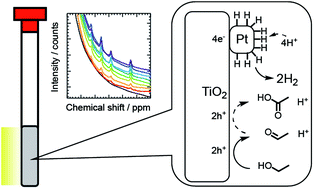
Understanding molecular processes at nanoparticle surfaces is essential for designing active photocatalytic materials. Here, we utilize nuclear magnetic resonance (NMR) spectroscopy to track photocatalytic hydrogen evolution using donor molecules and water isotopologues. Pt–TiO2 catalysts were prepared and used for isotopic hydrogen evolution reactions using alcohols as electron donors. 1H NMR monitoring revealed that evolution of the H2 and HD species is accompanied by the oxidation of donor molecules. The isotopic selectivity in the hydrogen evolution reaction gives rise to formal overpotential. Based on a comparison of the rates of hydrogen evolution and donor oxidation, we propose the use of ethanol as an efficient electron donor for the hydrogen evolution reaction without re-oxidation of radical intermediates.


 Please wait while we load your content...
Please wait while we load your content...