Metal chloride anion based ionic liquids: synthesis, characterization and evaluation of performance in hydrogen sulfide oxidative absorption†
Abstract
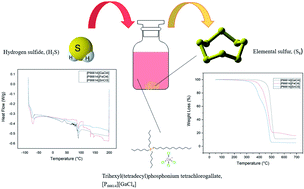
Three metal chloride anion based ionic liquids (MCABILs) were synthesized and characterized for high conversion of hydrogen sulfide (H2S). The MCABILs were synthesized via metathesis reaction and characterized by chemical spectroscopy such as FTIR, UV-Vis and CHNS elemental analysis. Then, the performance of those ionic liquids in H2S conversion was evaluated at various temperatures and atmospheric pressure. The results indicated remarkable efficiency of metal chloride anion based ionic liquids in H2S conversion. MCABILs managed to reach over 90% conversion efficiency at temperature as low as 50 °C, despite operating at atmospheric pressure. This occurs due to their high oxidation capability. The regeneration experiment indicated that all MCABILs can be recycled easily. Taken together, the research findings highlight the synthesis, characterization and high efficiency of MCABILs in promoting faster H2S conversion as a catalyst.


 Please wait while we load your content...
Please wait while we load your content...