Thermally stable mesoporous tetragonal zirconia through surfactant-controlled synthesis and Si-stabilization†
Abstract
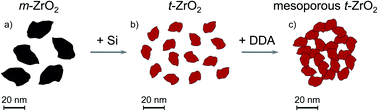
Thermally stable, highly mesoporous Si-stabilized ZrO2 was prepared by sol–gel-synthesis. By utilizing the surfactant dodecylamine (DDA), large mesopores with a pore width of ∼9.4 nm are formed. Combined with an NH3-treatment on the hydrogel, a high specific surface area of up to 225 m2 g−1 and pore volume up to 0.46 cm3 g−1 are obtained after calcination at 973 K. The individual contributions of Si-addition, DDA surfactant and the NH3-treatment on the resulting pore system were studied by inductively coupled plasma with optical emission spectrometry (ICP-OES), X-ray diffraction (XRD), N2 sorption, and transmission electron microscopy (TEM). Electron tomography was applied to visualize and investigate the mesopore network in 3D space. While Si prevents the growth of ZrO2 crystallites and stabilizes the t-ZrO2 phase, DDA generates a homogeneous mesopore network within the zirconia. The NH3-treatment unblocks inaccessible pores, thereby increasing specific surface area and pore volume while retaining the pore width distribution.


 Please wait while we load your content...
Please wait while we load your content...