Construction of sulfur-containing N-vinylimides: N-addition of imides to propargyl sulfonium salts†
Abstract
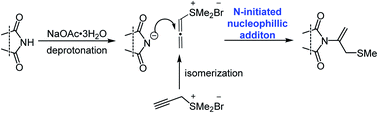
An N-addition reaction between imides and propargyl sulfonium salts was developed to afford sulfur-containing N-vinylimides with moderate to excellent yields. Under the activation of NaOAc·3H2O, imides could undergo deprotonation and propargyl sulfonium salts could isomerize to allenic sulfonium salts. The N-nucleophilic attack initiates the reaction and gives the desired products. Various imides, including arylimides, aliphatic imides and N-(arylsulfonyl) alkyl acylamides, and even bioactive saccharin, thalidomide and pomalidomide could provide organosulfur N-vinylimides compounds. The simple, mild and metal-free reaction conditions, the broad scope of substrates, gram-scale synthesis and convenient transformation embody the synthetic superiority of this process.


 Please wait while we load your content...
Please wait while we load your content...