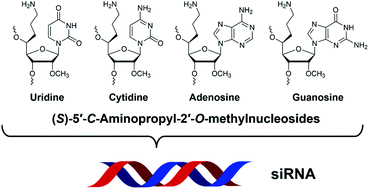
Synthesis, gene silencing activity, thermal stability, and serum stability of siRNA containing four (S)-5′-C-aminopropyl-2′-O-methylnucleosides (A, adenosine; U, uridine; G, guanosine; and C, cytidine)†
Abstract
Herein, we report the synthesis of (S)-5′-C-aminopropyl-2′-O-methyladenosine and (S)-5′-C-aminopropyl-2′-O-methylguanosine phosphoramidites and the properties of small interfering RNAs (siRNAs) containing four (S)-5′-C-aminopropyl-2′-O-methylnucleosides (A, adenosine; U, uridine; G, guanosine; and C, cytidine). The siRNAs containing (S)-5′-C-aminopropyl-nucleosides at the 3′- and 5′-regions of the passenger strand were well tolerated for RNA interference (RNAi) activity. Conversely, the (S)-5′-C-aminopropyl modification in the central region of the passenger strand decreased the RNAi activity. Furthermore, the siRNAs containing three or four consecutive (S)-5′-C-aminopropyl-2′-O-methylnucleosides at the 3′- and 5′-regions of the passenger strand exhibited RNAi activity similar to that of the corresponding 2′-O-methyl-modified siRNAs. Finally, it was observed that (S)-5′-C-aminopropyl modifications effectively improved the serum stability of the siRNAs, compared with 2′-O-methyl modifications. Therefore, (S)-5′-C-aminopropyl-2′-O-methylnucleosides would be useful for improving the serum stability of therapeutic siRNA molecules without affecting their RNAi activities.


 Please wait while we load your content...
Please wait while we load your content...